Distance dependence of the Electron-Nuclei Polarization Transfer
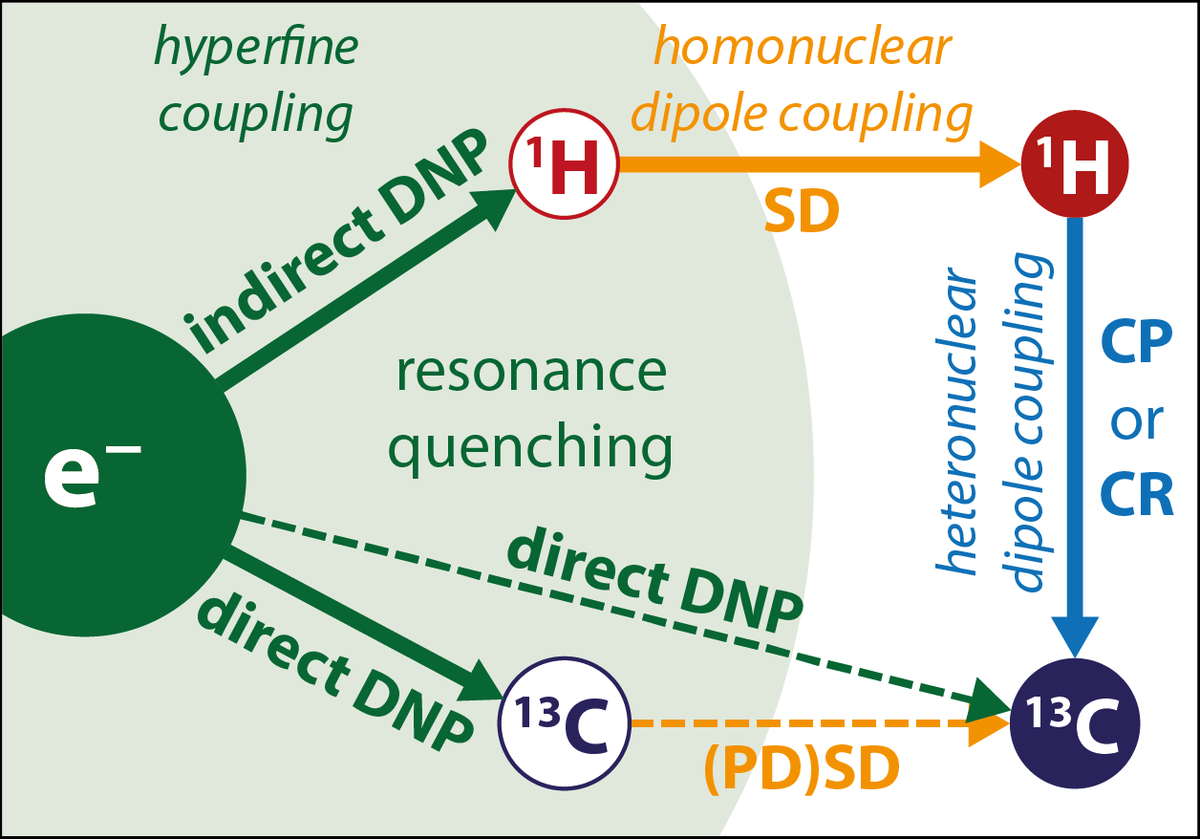
Despite the existing quantum mechanical knowledge for the description of easy spin-systems, the distance prediction, based on polarization transfer via DNP is very difficult. This lays in the variety of different effects that can influence the DNP transfer. The hyperfine coupling between electron- and nuclei-spins enables the transfer of polarization, but at the same time, couplings to other spins can interfere with the main transfer. In bigger systems, this fact leads to a complex scheme of different interactions between electrons and nuclei.
The knowledge about the DNP transfer rate as a function of electron-nuclei-distance and other parameters such as rates of relaxation and spin diffusion can bring an advantage for specific applications. For example, specific electron spin labeling of molecules or complexes could allow efficient distance determination via DNP. Moreover, selective NMR detection of labeled components in a mixture would be possible.
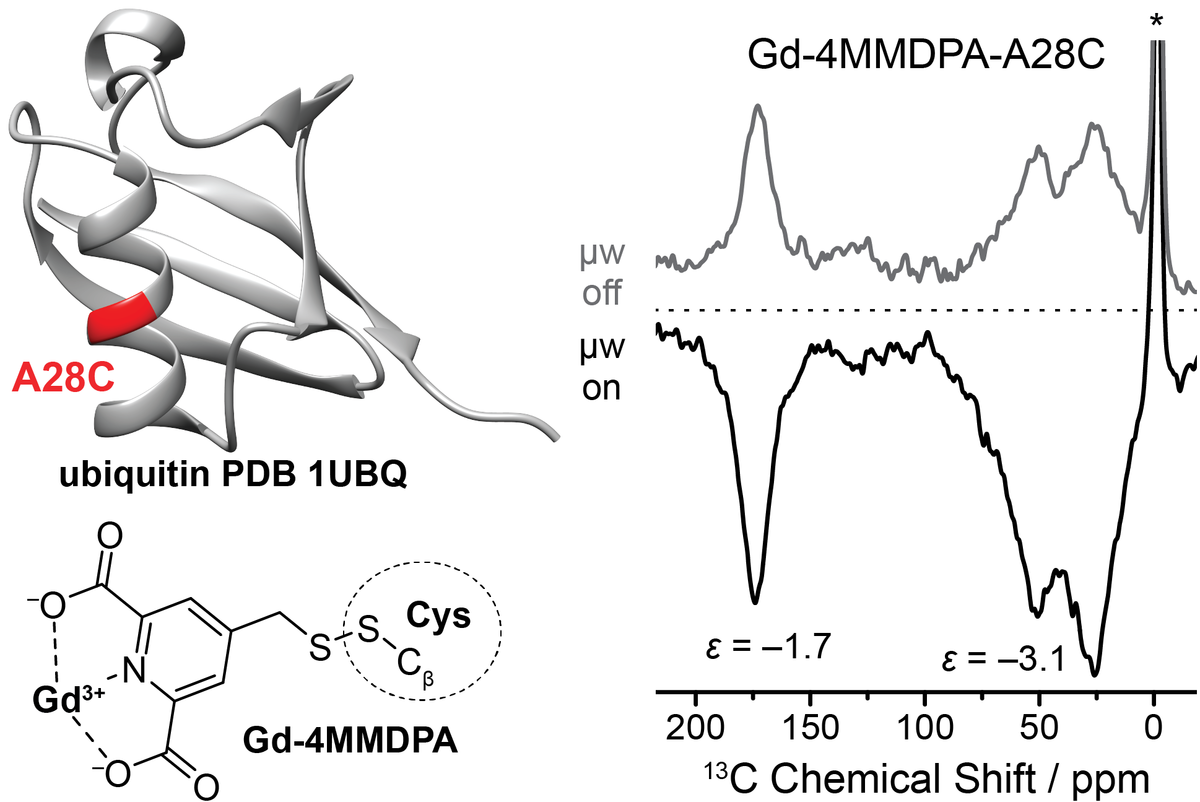
Direct DNP with Gd-spin labeling
This protein plays an important role in the decomposition of other proteins while apoptosis. During the protolyses, one or several ubiquitin molecules are bound to the target molecule by a specific covalent binding on the C-terminus of a lysine. This complex is then recognized by the proteasome and degraded. Because of its important function in cell ubiquitin is a very good understood model system that still offers many possibilities for the investigation of proteolysis with DNP and was therefore well-suited for our research. In one of our studies, we selectively changed the amino acid sequence on three different positions for a specific attachment of spin-labels at these postions. These tags were based on the Gd3+ chelate-complexes and were covalently bound to the mutation sites in the protein. We moreover could show, that these metal ions could be used as a polarizing agent for site-specific hyperpolarization in the protein.
Site-specific DNP in perdeuterated protein
In our recent work, we look at the influence of the proton concentration in the sample to the direct DNP in Gd-DOTA-ubiquitin. Due to the deuteration of the protein while the recombinant expression a precise variation change of isotope rate between protons and deuterons is possible. Through this variation, the spin-diffusion, as well as the cross-relaxation rate of 13C and 15N, can be affected. This allows us a significant increase in direct DNP enhancement. Further, we could show, that direct DNP can in principle be used for distance measurements between the electron and nuclei. These results will be published soon.